Acid or Base Experiment
Chemistry is such a fun science to explore, and one that you can often do in your own kitchen. With summer approaching, why not spend a few weeks exploring chemistry, using items you have around the house? Your kids will be thankful for the break from their core subjects, as the discussions are replaced with thoughts of atoms, chemical bonds, and the periodic table.
To inspire you, here’s a simple acid or base experiment that illustrates the acidity of household products in your home.
I’ve seen this experiment done online with a few liquids and red cabbage. However, I’m not a fan of cooking cabbage, nor do I have the time to boil it. Instead, here’s the same experiment, but using pH strips. They typically only cost a few dollars (and save my house from the smell of cabbage).
Materials needed:
- vinegar
- a can of any brand soda
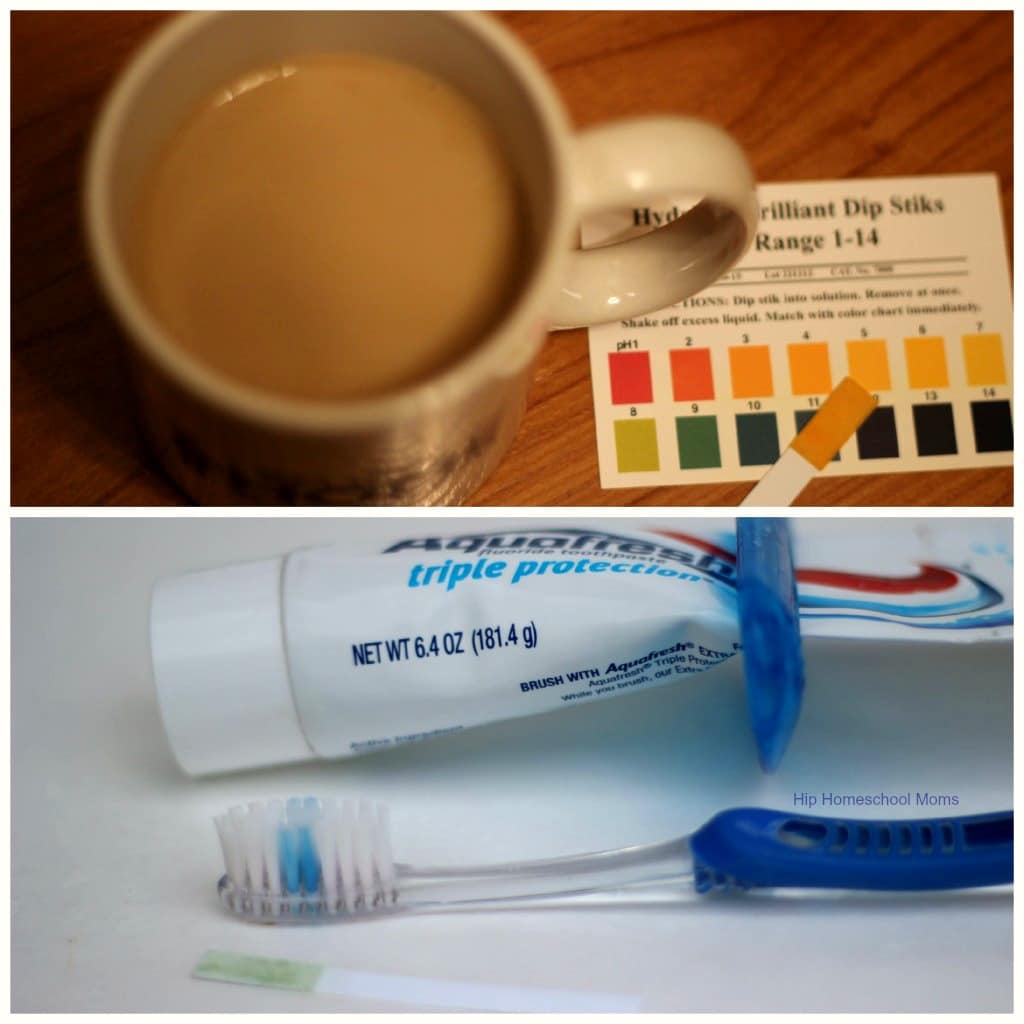
- black coffee
- milk
- water
- antacid tablets, crushed and dissolved in a small amount of water
- dishwasher gel
- beakers (or any small plastic cup or container)
- napkins
- pH strips
Vocabulary to know before the experiment:
- Acid
- Base
- Neutral
- pH scale
- litmus test
The Acid or Base Experiment:

1. Place each liquid into a clean beaker or cup. Label the beakers, so you can identify them after the experiment. You may also want to label the dipsticks.

2. Carefully place each dipstick into the liquid. The place it onto a napkin, in front of the liquid.

3. Compare the dipstick to the pH chart. Determine the pH.

4. After you’ve determined the pH of each liquid, sort the liquids from most acidic to least acidic.
Extensions:
- Have the kids smell the liquids, to determine what they are. Note: Normally, you would not smell a chemical. However, use your own judgement whether it would be safe for your kids to carefully smell these items.
- Ask the kids what they notice about the drinks. Are they acidic or basic? Since you are ingesting it, what effect do they think it will have on their bodies? Remind them of studies that show that drinking too many sodas (for adults, as few as 3) each day, can cause the enamel to be eaten off of their teeth.
- Want to see the effects of acids on your teeth? Try putting an egg in different liquids – such as vinegar, soda, and water. See what happens to the egg over the course of a few days.
- Is toothpaste acidic or basic? If many drinks are acidic, why is it so important to brush our teeth?
- What is the purpose of taking antacids? From our results, why would we take them?
- Test other liquids in your house. Here’s a few to consider:
- lemon juice, orange juice, or apple juice
- tea
- tomatoes
- washing soda (diluted in water)
- ammonia
- lye
- salt water
- drain cleaner, borax, and other household cleaning products (use caution, never allow children to smell or taste, and constant supervision!)
- milk of magnesia
- shampoo
- egg whites
- saline solution
- maple syrup
- oil-based salad dressings
- Gatorade and other drinks
- Combine two liquids and determine what the resulting pH level would be. For older kids, predict the outcome of an acid and a base, two acids, or two bases.
Want to learn more about Chemistry? Try out Bright Ideas Press Christian Kids Explore Chemistry, which includes an explanation of basic facts about Chemistry, and adds in simple science experiments that illustrate the science behind each topic. This activity goes great after finishing unit 3 or after chapters 16-17, about acids and bases. Christian Kids Explore Chemistry is $39.99, and is designed for grades 4-8. However, we’ve easily used it for lower grades.
What other chemistry experiments have you completed in your home? Have you ever done an acid or base experiment as a part of your homeschool?
*A special thanks to Bright Ideas Press, who donated this book to one of the team members, and inspired this activity.







