Tampons Being Recalled – December 2018
We have another recall to share with our community … this one by Kimberly-Clark. They have announced a voluntary product recall of its U by Kotex® Sleek® Tampons, Regular Absorbency. See details below.
What is Recalled?
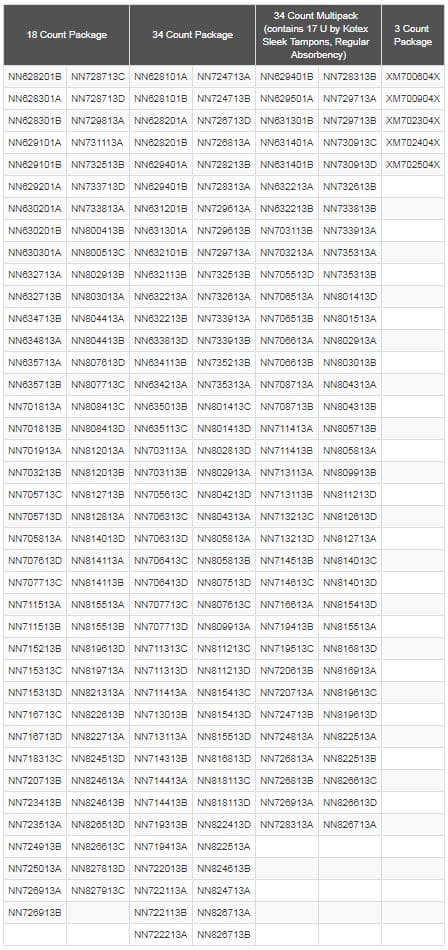
The recall is limited to specific lots of U by Kotex® Sleek® Tampons, Regular Absorbency, that were manufactured between October 7, 2016 and October 16, 2018 and distributed between October 17, 2016 and October 23, 2018. Consumers can identify this product by looking for specific lot numbers found on the bottom of the package. A full list of recalled lot numbers is available on the U by Kotex® website. Retailers have been alerted to remove the recalled lot numbers from shelves and post a notification in their stores.
Per Kimberly-Clark, no other U by Kotex-branded products are subject to this recall.
Why is it Recalled?
Kimberly-Clark has received reports from consumers of the U by Kotex® Sleek® Tampons, Regular Absorbency, unraveling and/or coming apart upon removal, and in some cases causing users to seek medical attention to remove tampon pieces left in the body. There also have been a small number of reports of infections, vaginal irritation, localized vaginal injury, and other symptoms.
Where was the product sold?
These tampons were sold throughout the United States and Canada for a quality-related defect that could impact the performance of this product.
What to do?
Any consumer with the impacted U by Kotex® Sleek® Tampons, Regular Absorbency, in their possession should stop using the product immediately and promptly contact Kimberly-Clark’s Consumer Service team at 1-888-255-3499 between 7:30 a.m. – 7:00 p.m. Central Time, Monday through Friday, for information regarding this recall. Consumers who experience vaginal injury, (pain, bleeding, or discomfort), vaginal irritation (itching or swelling), urogenital infections (bladder and/or vaginal bacterial and/or yeast infections), or other symptoms such as hot flashes, abdominal pain, nausea, or vomiting following use of the impacted product should seek immediate medical attention.
U.S. health care professionals and consumers may report adverse reactions or quality problems they may experience using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program either online, by regular mail or by facsimile to 1-800-FDA0178.
Canadian health care professionals and consumers may report device-related incidents directly to Health Canada by completing a Health Product Complaint Form online.







